-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
-
Flow Cytometry Reagents
- Immunoassay Reagents
- Single Cell Multiomics Reagents
-
Cell Preparation
-
Functional Assays
-
Microscopy and Imaging Reagents
- Western Blotting And Molecular Reagents
- Cell Preparation Separation Reagents
- Functional Cell Based Reagents
- Microscopy Imaging Reagents
- Single Cell Multiomics Reagents
- Single Cell Multinomics Reagents
-
Account Support
-
Account FAQs
- Account FAQ Answer 1
- Account FAQ Answer 2
- Account FAQ Answer 3
- Account FAQ Answer 4
- Account FAQ Answer 5
- Account FAQ Answer 6
- Account FAQ Answer 7
- Account FAQ Answer 8
- Account FAQ Answer 9
- Account FAQ Answer 10
- Account FAQ Answer 11
- Account FAQ Answer 12
- Account FAQ Answer 13
- Account FAQ Answer 14
- Account FAQ Answer 15
- Account FAQ Answer 16
- Account FAQ Answer 21
- Create Account
- Manage Account Settings
-
PrivacyPolicy
-
Terms and Conditions
-
Account FAQs
-
- Account FAQ Answer 1
- Account FAQ Answer 2
- Account FAQ Answer 3
- Account FAQ Answer 4
- Account FAQ Answer 5
- Account FAQ Answer 6
- Account FAQ Answer 7
- Account FAQ Answer 8
- Account FAQ Answer 9
- Account FAQ Answer 10
- Account FAQ Answer 11
- Account FAQ Answer 12
- Account FAQ Answer 13
- Account FAQ Answer 14
- Account FAQ Answer 15
- Account FAQ Answer 16
- Account FAQ Answer 21
- Japan (English)
- Japan (Japanese)
-
Change country/language
Old Browser
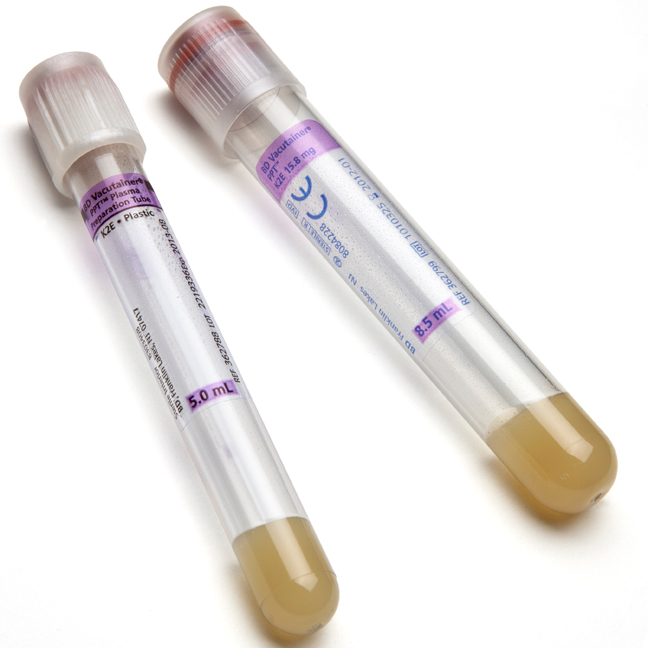
BD Vacutainer® PPT™ Plasma Preparation Tube - Paper Label

Regulatory Statusの凡例
Becton, Dickinson and Companyの書面による明示的な許諾を得た使用以外での製品の使用は固く禁じられています。
説明
BD Vacutainer® PPT™ is a closed system allowing separation and storage of undiluted EDTA plasma in the primary blood collection tube. It is intended for the purposes of molecular diagnostic testing.
PPT™ is an evacuated, sterile blood collection tube that contains an inert gel and spray-dried K2EDTA anticoagulant for achieving plasma separation. When the sample-filled tube is subjected to centrifugation, the gel migrates and forms a physical barrier between the plasma and most of the cellular elements. Since the tube uses spray-dried K2EDTA as opposed to liquid additives, the plasma obtained is undiluted.
| Tube Material | Plastic |
| Tube Size | 16x100 mm |
| Draw Volume | 8.5 mL |
| Closure Type | BD Hemogard™ |
| Closure Color | Pearl White |
| Label | Paper |
| Cell Separator | Yes (Gel) |
| Anticoagulant | K2EDTA |
| Sterilization | Radiation , 10-6 SAL , ISO11137-1 |
- BD White Paper VS8188: Evaluation Of The Effect Of Specimen Handling Conditions In BD Vacutainer® PPT On The Stability Of HIV-1 Viral Load Using Roche Cobas® Ampliprep/ Cobas® Taqman® HIV-1 Test, 2010.
- BD White Paper VS8189: Evaluation of Specimen Handling Conditions in BD Vacutainer® Plasma Preparation Tube HIV-1 Viral Load as Measured by the Abbott RealTime HIV-1 Assay.
- Evaluation of the VACUTAINER® Brand PPT™ Tubes for HCV Viral Load Testing.
- Determination of the Effect of Freezing BD Vacutainer® PPT™ Plasma in situ on Hepatitis C (HCV) Viral Loads as Measured by the Roche COBAS® TaqMan® HCV ASR.
- Determination of the Effect of Freezing BD Vacutainer® PPT™Plasma in situ on Hepatitis B (HBV) Viral Loads Using the Roche COBAS® TaqMan® HBV RUO Assay.
調製と保管タイトルテキスト
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
BD-23290
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.