Old Browser
Overview
Built on more than 45 years of BD experience and leadership in flow cytometry and multicolor analysis, the BD FACSCanto™ II Flow Cytometry Systems deliver reliable performance, accuracy and ease-of-use for today's busy clinical laboratories.
Get more information from the BD FACSCanto™ II System brochure.


Features
The BD FACSCanto™ II Flow Cytometer automates many features to help streamline the process and reduce hands-on time for operators

Keeps operations running efficiently
- Quality control and single-tube instrument setup help operators rapidly learn how to run routine clinical applications, improving the reliability and consistency of results
- BD FACS™ Clinical Software automates setup, compensation analysis and quality control for predefined clinical applications
Innovations to improve care
The BD FACSCanto™ II System features a fixed-alignment flow cell in the fluidics system that minimizes startup time and improves reproducibility. To increase sensitivity and resolution for each color in a multicolor assay, a patented optical design maximizes signal detection.
The BD FACSCanto™ II System’s high sensitivity delivers accurate results

- Innovative designs for both excitation and collection optics to reduce excitation losses so that more information can be gained from each sample
- Fixed alignment of lasers from day-to-day and from experiment-to-experiment without user intervention
- Patented detector arrays to maximize signal retention
- Unique reflective design that guides light to their target detectors to improve sensitivity
Get more information on the optics from the BD FACSCanto™ II System brochure.
PERFORMANCE
The BD FACSCanto™ II System is a powerful and reliable cell analyzer for busy, best-in-class laboratories

The BD FACS™ Loader instrument option enables walkaway sample introduction to improve productivity
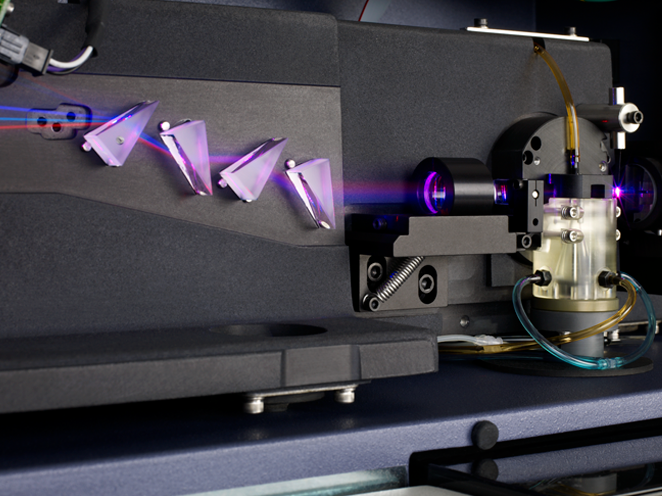
Unique reflective design improves sensitivity

Innovations in the fluidics system deliver optimal system performance
APPLICATIONS
BD FACSCanto™ Clinical Software on the BD FACSCanto™ II System enables automatic gating
BD FACSCanto™ clinical software automates setup, compensation analysis, and quality control for predefined clinical applications. It includes specific application modules optimized for use with specific IVD reagent kits. The modules feature automated gating, calculations, and report generation to deliver a consistent, reproducible, and standardized analysis.
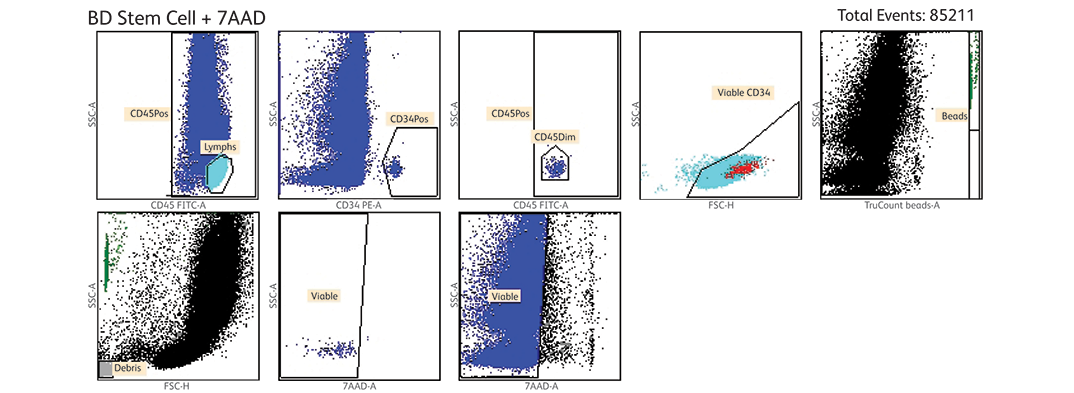
Take advantage of the protocols available for various applications, such as lymphocyte and stem-cell enumeration, HLA immunophenotyping and more.
BD FACSCanto™ clinical software is an IVD-cleared product for enumerating lymphocyte subsets of samples stained with BD Multitest™ 4- and 6-color reagents.
Activation Marker (BD Multitest™ CD8/CD38/CD3/Anti-HLA-DR) Software module for BD FACSCanto™ clinical software is available for supporting the acquisition and analysis of samples stained with Multitest CD8/CD38/CD3/Anti-HLA-DR on the BD FACSCanto™ II flow cytometers.
A BD® Stem Cell Enumeration Software module for BD FACSCanto™ clinical software offers a validated analysis software, designed to meet productivity and quality requirements of clinical laboratories for the BD® Stem Cell Enumeration Kit.
A BD® HLA-B27 Software module for the BD FACSCanto™ II system provides a complete system for rapid detection of HLA-B27 antigen, which is clinically relevant to the evaluation of seronegative spondyloarthropathies.
-
Brochures
-
Filter Guide
-
Technical Specifications
-
Product Information Sheets
-
Quick Reference Guides
-
User Guides and Manuals
-
BD™ HLA-B27 Application Guide for BD FACSCanto™ Flow Cytometers
-
BD FACSCanto™ II HTS User Guide
-
BD™ Stem Cell Enumeration Application Guide
-
BD OneFlow™ Instrument Setup Guide
-
BD OneFlow™ LST Application Guide
-
BD OneFlow™ B Cell Diseases Application Guide
-
BD OneFlow™ Plasma Cell Disorder Application Guide
-
BD OneFlow™ ALOT Application Guide
-
Webinars
-
Application Notes
-
Technical Documents
-
Frequently Asked Questions
-
Protocols

Request a Quote
Please fill in the following information and we will get in touch with you regarding your query.
![]()
BD FACSCanto II ™ flow cytometers are Class 1 laser products.
BD FACS™ Loader instrument and BD FACSCanto II ™ are CE marked in compliance with the European In Vitro Diagnostic Medical Device Directive 98/79/EC.
BD™ HLA-B27 Kit, BD™ Stem Cell Enumeration Kit, BD Multitest™ IMK Kit, BD Multitest™ 6-color TBNK Reagent with BD Trucount™ Tubes, BD Multitest™ 6-color TBNK and BD Multitest™ CD8/CD38/CD3/Anti-HLA-DR are CE marked in compliance with the European In Vitro Diagnostic Medical Device Directive 98/79/EC.
Cy™ is a trademark of GE Healthcare. Cy™ dyes are subject to proprietary rights of GE Healthcare and Carnegie Mellon University, and are made and sold under license from GE Healthcare only for research and in vitro diagnostic use. Any other use requires a commercial sublicense from GE Healthcare, 800 Centennial Avenue, Piscataway, NJ 08855-1327, USA.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.


