Old Browser
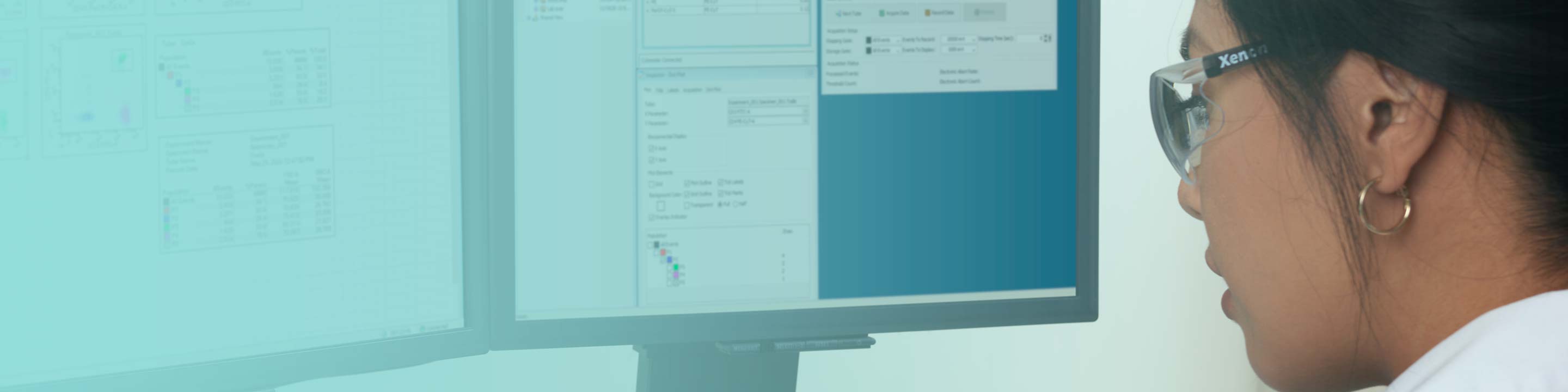
BD FACSDiva™ Software
Expanding confidence and flexibility in today’s integrated laboratory
![]()
BD FACSDiva™ CS&T IVD Beads, BD OneFlow™ products, BD FACSCanto™ II Flow Cytometer and its optional BD FACS™ Loader are in vitro diagnostic medical devices bearing a CE mark.
BD FACSCelesta™, BD FACSAria™ III, BD FACSAria™ Fusion, BD LSRFortessa™, BD LSRFortessa™ X-20, BD™ High Throughput Sampler, BD FACS™ Accudrop Beads, BD Phosflow™ products and BD FACSDiva™ CS&T Research Beads are for Research Use Only. Not for use in diagnostic or therapeutic procedures.
BD Flow Cytometers are Class 1 Laser Products.
BD FACSAria™ II and BD™ LSR II are discontinued.
BD FACSCanto™ Flow Cytometer (10-color configuration) is discontinued in EU.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.