Old Browser
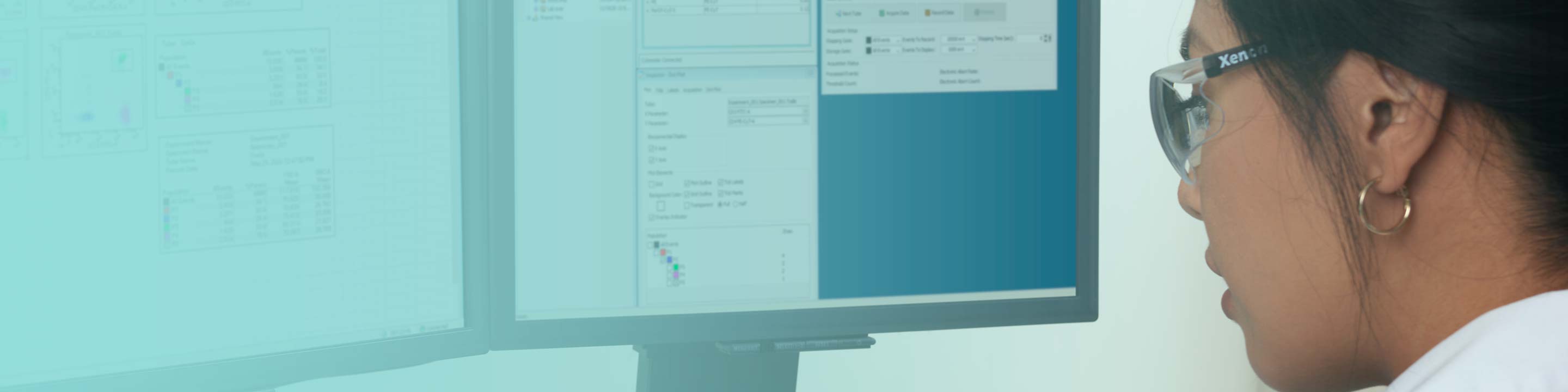
BD FACSuite™ Application
Acquisition and analysis application that is robust and reliable with features enabling US FDA 21 CFR Part 11 compliance
For Professionals in Research and Clinical Diagnostics
BD FACSLyric™ Flow Cytometers are Class 1 Laser Products. The BD FACSLyric™ Flow Cytometer is for In Vitro Diagnostic Use with BD FACSuite™ Clinical Application for up to six colors. The BD FACSLyric™ Flow Cytometer is for Research Use Only with BD FACSuite™ Application for up to 12 colors. Not for use in diagnostic or therapeutic procedures.
Cy is a trademark of Global Life Sciences Solutions Germany GmbH or an affiliate doing business as Cytiva.
Refer to manufacturer's instructions for use and related User Manuals and Technical Data Sheets before using this product as described.
Comparisons, where applicable, are made against older BD technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.