-
Account Support
-
Contact
-
Technicalsupport
-
TrainingPage
-
TextAndDownloadTest
-
Protocol Details
-
Protocol Library Component
-
Multi-coloredtablecomponent
-
BDB Protocol Library
-
First Protocol Detail
-
Second Protocol Detail
-
Third Protocol Detail
-
Fourth Protocol Detail
- TrainingDetails
-
RewardsLandingPage
-
TrainingLandingPage
-
Technical Support
-
Bulletpointspage
-
PrivacyPolicy
-
Freeformcomponent
-
Account FAQs
-
Contact
Old Browser
BSB Protocol FAQ
BD® CompBead Particles are coated with anti-immunoglobulin, which can bind most fluorescently conjugated antibodies, acting as a surrogate stained cell. They can be used easily and produce great results by resolving peaks for each fluorochrome. The limitation of any bead control, however, is that since they are not actual stained cells the spillover values can be different from similarly stained cells.
BD® CompBead Particles are species and kappa chain specific. Make sure your BD® CompBead Particles are compatible with both the species and light chain of the reagent you are testing. We provide BD® CompBead Particles compatible with a range of species and sizes (Cat. No. 552843, 552844, 552845; BD® CompBead Plus Particles, Cat. No. 560497, 560499)
No, the emission spectrum (% spillover) of the compensation control reagent should be identical to the reagent used in the experiment. Even similar fluorochromes such as FITC, Alexa Fluor™ 488 and GFP have different emission spectra, which can lead to different spillover values for each dye. This also applies to live/dead dyes like PI, 7-AAD and the BD Horizon™ Fixable Viability Stains.
Different lots of tandem dyes can have significantly different spillover values. Therefore, we recommend lot-specific compensation for tandem dyes. There should be a compensation control for each tandem dye in your experiment.
We recommend treating compensation controls the same way as your sample was prepared. This includes the same-day preparation, using the same reagents (e.g., same perm buffer, using BD Horizon™ Brilliant Stain Buffer (BSB) for compensation controls), as well as the same incubation and storage time. Matrices generated at different times using the same reagents can have differences that affect the compensation of your samples.
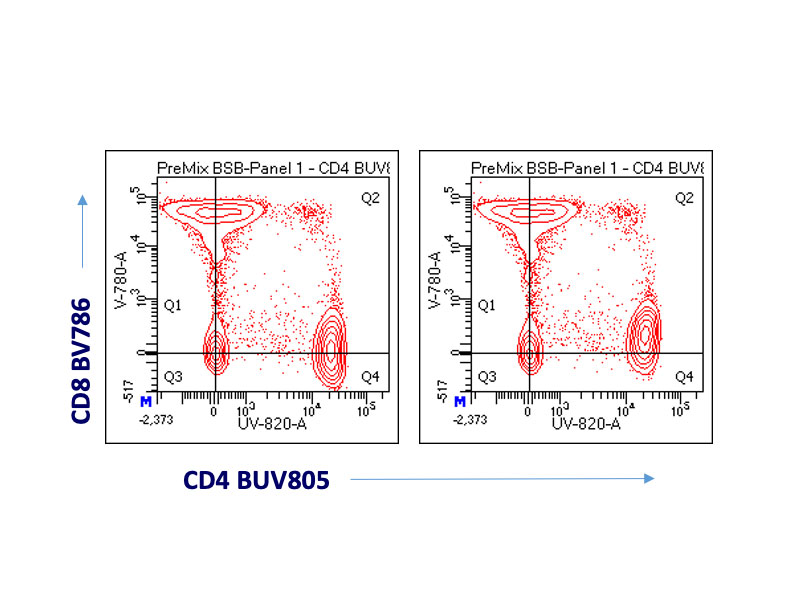
A cocktail of CD4 BD Horizon Brilliant™ UV 805 (BUV805) and CD8 BD Horizon Brilliant Violet™ 786 (BV786) Antibodies was either prepared with BSB or pre-incubated for two hours with BSB (“pre-mix”). These cocktails were used to stain frozen and thawed PBMCs, which were then washed, fixed and acquired. The plot on the left used compensation calculated with reagents pre-incubated with BSB for two hours. The plot on the right used compensation calculated with compensation controls prepared and acquired immediately. Notice the under-compensation using fresh reagents compared to controls treated the same as the sample.
Yes. BD Horizon™ Brilliant Stain Buffer (BSB) is used anytime two or more BD Horizon Brilliant™ Dyes are used in the same experiment to prevent staining artefacts from dye-dye interactions. When using BSB in a multicolor experiment, it is best to use BSB in single-color compensation control tubes as well. The buffer is compatible with BD® CompBead Particles, however, it has not been tested with compensation beads from other vendors.
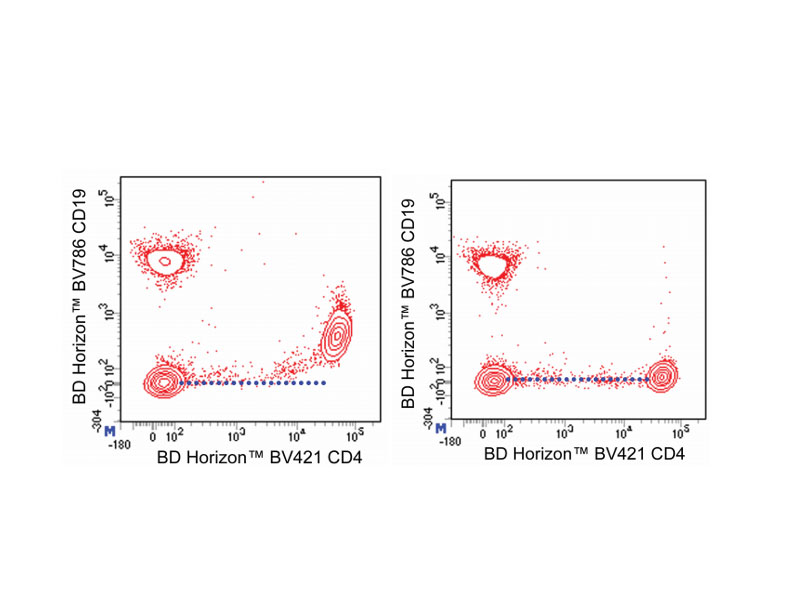
Either BD Horizon™ Brilliant Stain Buffer or BD Horizon™ Brilliant Stain Buffer Plus should be used in multicolor immunofluorescent staining and flow cytometric analysis when using two or more BD Horizon Brilliant™ Reagents. Whole human blood was stained with BD Horizon Brilliant Violet™ 421 (BV421) Mouse Anti-Human CD4 and BD Horizon™ BV711 Mouse Anti-Human CD19 Antibodies. Erythrocytes were lysed with BD FACS™ Lysing Solution (Cat. No. 349202). Two-color flow cytometric contour plots showing the correlated expression of CD4 versus CD19 were derived from gated events with the forward and side light-scatter characteristics of intact lymphocytes. Staining cells in the presence of BD Horizon™ Brilliant Stain Buffer (Cat. No. 563794/566349; right plot) restores the major lymphocyte populations to their expected fluorescent staining patterns (dotted lines) when compared with cells stained without a Brilliant Stain Buffer (left plot). Flow cytometric analysis was performed using a BD LSRFortessa™ Cell Analyzer System.
Voltages for fluorescent parameters must be maintained during compensation acquisition but FSC and SSC can be changed. This is especially useful when combining cells and beads for compensation, which may not lie at the same place on FCS/SSC plots.
Typically, when using cells to create compensation, a universal negative is used to define the negative population fluorescence. When combining cells and beads, for the tubes containing single-stained cells as compensation controls, create an autointerval gate (P3) around the negative population in each fluorescence histogram. This often applies when using beads for all fluorochromes except 7-AAD or viability dyes when using cells.
Calculated compensation will only be correct up to the brightness of your positive compensation control. If the compensation control is off scale, we recommend titrating down the amount of antibody added to the control, rather than adjusting the PMTV.
These dyes do not work optimally with BD® CompBead Particles, and spillover values for these dyes evaluated with BD® CompBead Particles may not provide correct compensation for cells. Single-stained cellular controls may give more accurate compensation results.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.