Old Browser
{{countryName}}

BD OneFlow™ LST
Overview
The BD OneFlow™ LST is part of the innovative BD OneFlow™ Solution, a comprehensive set of reagents, setup beads, protocols and assay templates to reproducibly set up the flow cytometer and stain, acquire and analyze patient specimens for immunophenotyping of normal and aberrant cell populations. It is built on the research and validation work of the EuroFlow™ Consortium on the characterization of hematological malignancies for improved accurate diagnosis.1*
The BD OneFlow™ LST is intended for flow cytometric immunophenotyping of normal and aberrant mature lymphocyte populations of B, T and NK cell lineages in peripheral blood, bone marrow and lymph nodes.
Other reagents belonging to the BD OneFlow™ Solution are BD OneFlow™ B-CLPD T1, BD OneFlow™ PCST, BD OneFlow™ PCD.
FEATURES
The BD OneFlow™ LST:
- Is a preconfigured single-use, ready-to-use 8-color 12-antibodies reagent that is provided in a single-test tube format
- Can guide the need for further analysis as a screening tube in combination with panel(s), such as the BD OneFlow™ B-CLPD-T1, specifically designed for the classification of different forms of malignancies (B, T or NK)
- Is available in the 20 test/box size (4 pouches of 5 tubes each)
- Allows for easy visual identification as dark blue color-coded boxes, pouches and tubes
The BD OneFlow™ Reagents increase efficiency by simplifying instrument standardization, reducing the time to prepare the instrument as well as the technical burden and training needs.2
For BD FACSLyric™ Flow Cytometer users:
- BD® CS&T IVD Beads standardize setup and monitoring for consistent performance and ensure reproducibility through Universal Setup
- BD® FC Beads (7-Color, 5-Color and 2-Color Kits) support consistency of results, eliminating the need for using cells for compensation
- BD® CS&T Beads and BD® FC Beads kits necessary for instrument setup and compensation can be used for other CE-IVD assays on the BD FACSLyric™ Flow Cytometer
For BD FACSCanto™ II Flow Cytometer users:
- BD FACSDiva™ CS&T IVD Beads standardize setup and monitoring for consistent performance and ensure reproducibility with Application Settings
- BD OneFlow™ Setup Beads ensure data accuracy and reproducibility by providing assay-specific target values, as per EuroFlow™ SOPs3
- Specifically designed BD® FC Beads eliminate the need for using single-vial reagents and cells for compensation
The BD OneFlow™ LST reagent composition
| Antibody | Fluorochrome | Clone | Target Populations |
|---|---|---|---|
| CD45 | BD Horizon V500-C | 2Dl (anti-HLe-1) | Mature lymphocytes, B-cell precursor |
CD19 | PE-Cy7 | SJ25-C1 | B-cells, and NK-cells by exclusion |
| CD20 | BD Horizon V450 | L27 | B-cells, and NK-cells by exclusion |
| Anti-Lambda | FITC | 1-155-2 | Normal and clonally expanded B cells |
| Anti-Kappa | PE | TB28-2 | Normal and clonally expanded B Cells |
| CD38 | APC-H7 | HB7 | Plasma cells and B-cell precursors, Lymphoid malignancies, NK cells |
| CD3 | APC | SK7 | T cells, B- and NK-cells by exclusion |
| CD4 | BD Horizon™ 450 | SK3 (Leu-3a) | T cell subpopulations |
| CD8 | FITC | SK1 (Leu-2a) | T cell subpopulations |
| CD5 | PerCP-Cy5.5 | L17F12 | T cell subpopulations |
| Anti-TCR-1 | PE-Cy7 | 11F2 | T cell subpopulations |
| CD56 | PE | MY31 (Leu-19) | NK Cells |
The EuroFlow antibody panel article1* has a full description of the utility of the antibodies chosen for the BD OneFlow™ LST.
APPLICATIONS
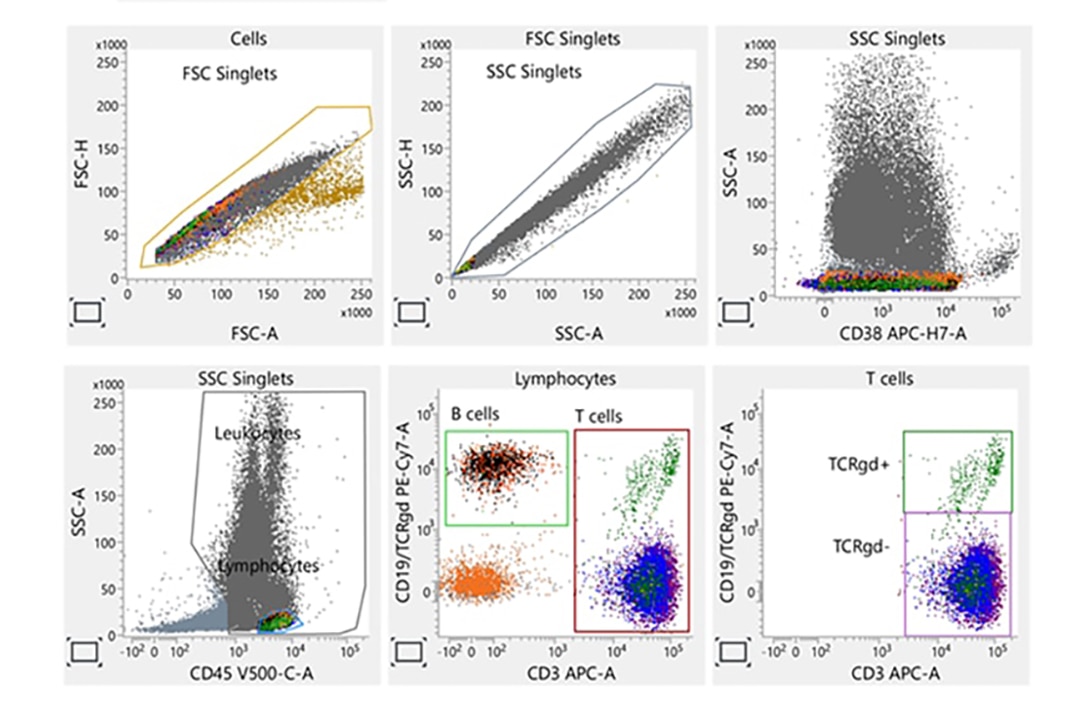
Laboratory report of a normal bone marrow specimen stained with the 8-color 12-antibody BD OneFlow™ LST single-test reagent acquired on the BD FACSLyric™ Flow Cytometer with BD FACSuite™ Clinical Application v1.4 for the identification of normal and aberrant mature lymphocyte populations of B, T and NK cell lineages.
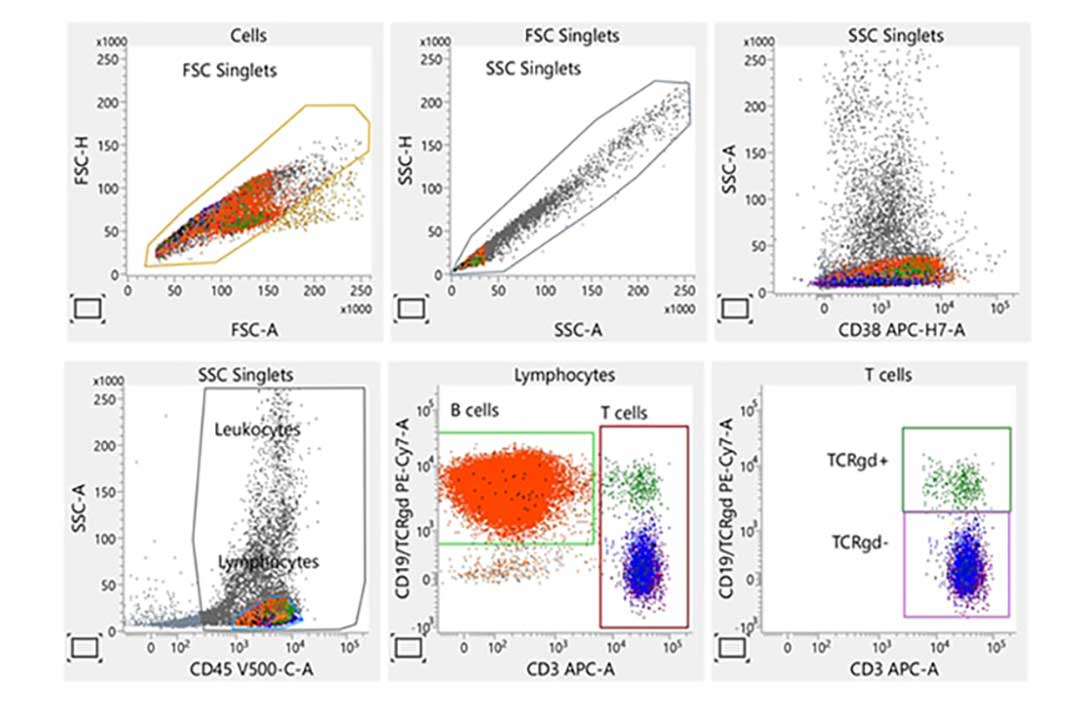
Laboratory report of an abnormal bone marrow specimen stained with the 8-color 12-antibody BD OneFlow™ LST single-test reagent acquired on the BD FACSLyric™ Flow Cytometer with BD FACSuite™ Clinical Application v1.4 for the identification of normal and aberrant mature lymphocyte populations of B, T and NK cell lineages.

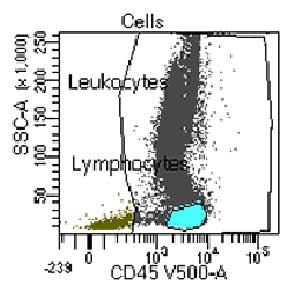
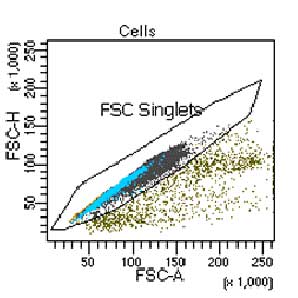
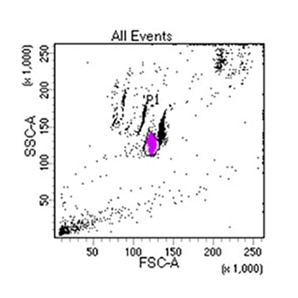
Supporting Evidence
The EuroFlow “LST detected aberrant B-, T-, or NK-cells immunophenotypes in 149/150 (99.4%) of B-CLPD and in 78/83 (94%) of T/NK-CLPD with an overall frequency of 97.4%."1*
Results from the clinical trial study show that the BD OneFlow™ LST in combination with the BD OneFlow™ B-CLPD T1 gave 100% (101 of 101) overall agreement in classifying patients as having CLL (54 of 54 concordant) and in identifying patients with other B cell chronic lymphoproliferative diseases (47 out of 47 concordant) when compared with the EuroFlow system. Furthermore, interpretation of the BD OneFlow™ Solution results was highly concordant to diagnostic truth.
References
- van Dongen JJM, Lhermitte L, Böttcher S, et al. on behalf of the EuroFlow Consortium (EU-FP6, LSHB-CT-2006-018708). EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 2012;26(9): 1908-1975. doi: 10.1038/leu.2012.120
- Moloney E, Watson H, Barge D, et al. Efficiency and health economic evaluations of BD OneFlow™ Flow Cytometry Reagents for diagnosing chronic lymphoid leukemia. Cytometry B Clin Cytom. 2019;96(6):514-520. doi: 10.1002/cyto.b.21779
- Kalina T, Flores-Montero J, van der Velden VHJ, et al. EuroFlow standardization of flow cytometer instrument settings and immunophenotyping protocols. Leukemia. 2012;26(9):1986-2010. doi: 10.1038/leu.2012.122
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.