Old Browser
BD FACSuite™ Application
Acquisition and analysis application that is robust and reliable with features enabling 21 CFR Part 11 compliance
Overview
The software that controls the BD FACSLyric™ Flow Cytometry System comprosies two applications, BD FACSuite™ Clinical Application and BD FACSutie™ Application. Both applications provide an easy-to-use interface for data acquisition and analysis with the BD FACSLyric™ System.
The BD FACSuite™ Clinical Application supports BD IVD assays with predefined assay templates.
The BD FACSuite™ Application is designed for user-defined assays and collaboration through the unique assay transfer feature, providing simple, reproducible and transferrable assay setup facilitating instrument-to-instrument and site-to-site standardization.
Functions included in both applications, including password protection, audit trail, electronic signatures and IQ/OQ procedures, assist in supporting 21 CFR Part 11 compliance and electronic record integrity.


The BD FACSuite™ Clinical Application provides a streamlined workflow, predefined assay templates for BD IVD assays, and comprehensive reporting
A streamlined workflow
BD® CS&T Beads are used to check cytometer performance and automatically make adjustments, ensuring consistent instrument setup from day to day. They are also used to perform comprehensive checks during system initialization and setup, including performance QC; verify system performance; and trigger the automated laser alignment process if required. This functionality is enabled by the BD FACSuite™ Clinical Application and ensures the entire setup process can be performed without manual intervention.
Predefined assay templates and comprehensive reporting
Assay modules with predefined templates optimized for use with specific BD IVD reagent kits can be installed on the BD FACSuite™ Clinical Application. These templates feature automated gating, calculations and report generation.


Predefined reports for BD IVD assays include laboratory, physician and supplemental reports and make reporting results fast and easy. Results can be easily exported to a laboratory information system (LIS) through the BD FACSLink™ LIS Interface.
The BD FACSuite™ Application provides a streamlined workflow and automated standardization enabling collaboration
A streamlined workflow
BD® CS&T Beads are used to perform comprehensive checks during system initialization and setup, including performance QC; verify system performance; ensure that detector settings are optimal; and trigger the automated laser alignment process if required. This functionality enabled by the BD FACSuite™ Application ensures the entire setup process can be performed without manual intervention.
Spillover value measurement is performed every 60 days using predispensed dried BD® FC Beads. Spillover values are maintained within the software and updated automatically with PMTV changes.
Standardization and collaboration
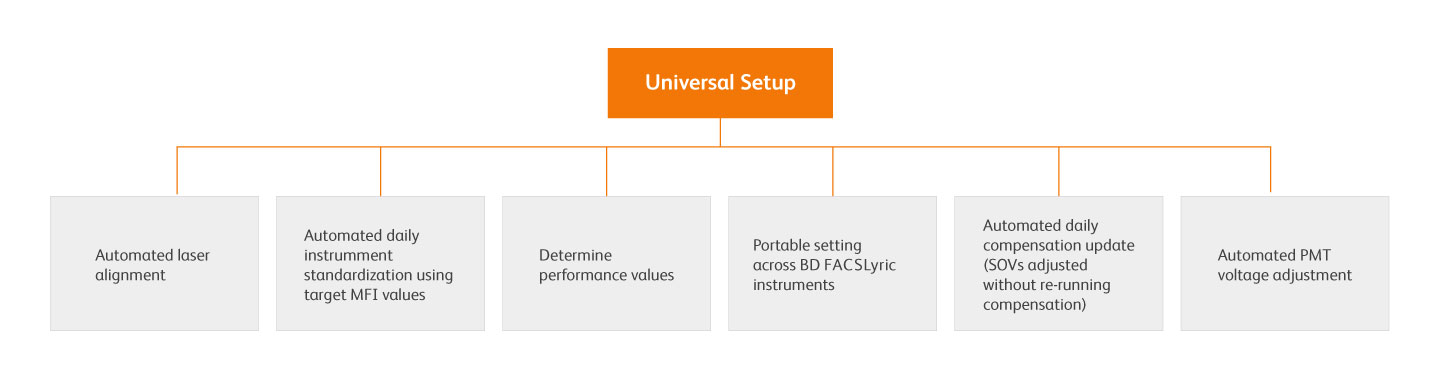
Universal setup is an automated system that establishes and maintains instrument MFI target values on a single instrument using the BD FACSuite™ Application, BD® CS&T Beads and BD® FC Beads, allowing for reproducible and consistent results within and between BD FACSLyric™ instruments over time.
| Blue Laser | %CV |
| FITC | 4.2 |
| PE | 4.4 |
| PE-Cy7 | 4.8 |
| PerCP | 10.2 |
| PerCP-Cy5.5 | 7.9 |
| Red laser | %CV |
| APC | 5.3 |
| APC-Cy7 | 4.1 |
| APC-H7 | 3.9 |
| APC-R700 | 4.2 |
| Violet laser | %CV |
| BD Horizon™ V450 | 11.1 |
| BD Horizon™ V500 | 11 |
| BD Horizon™ BV605 | 13.6 |
| BD Horizon™ BV711 | 7.5 |
| BD Horizon™ BV786 | 15.3 |
Table 1. Between-Instrument reproducibility of ytarget MFI values on the BD FACSLyric
Lyse/wash assay settings were imported across 15 instruments to show effects of standardization on beads. The CVs of the fluorescence intensity across all channels varies by less than 15.3%. (Figure 5) Daily QC with one lot of BD CS&T beads was run on fifteen BD FACSLyric instrument. The MF1 of positive populations was measeured for all parameters across all instruments. The %CV is shown.
The data for this internal study was acquired using BD FC beads across 15 Instruments. Greater between-instrument variability could be observed when running biological samples, when using non-BD reagents or when comparing fewer instruments.
Bead Lot Files

BD FACSLyric™ Flow Cytometers are Class 1 Laser Products. The BD FACSLyric™ Flow Cytometer is for In Vitro Diagnostic Use with BD FACSuite™ Clinical Application for up to six colors. The BD FACSLyric™ Flow Cytometer is for Research Use Only with BD FACSuite™ Application for up to 12 colors. Not for use in diagnostic or therapeutic procedures.
Cy is a trademark of Global Life Sciences Solutions Germany GmbH or an affiliate doing business as Cytiva.
23-23321-00
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.