Old Browser
Overview
The BD FACSDuet™ Sample Preparation System provides a powerful new level of performance to move the pace of your lab forward. The system:
- Is designed to complement and physically integrate with the BD FACSLyric™ Flow Cytometer
- Provides a complete walkaway sample-to-answer solution
- Drives productivity and accuracy in your results through its automation protocol, minimizing manual intervention
- Supports the labs in being compliant with ISO-15189 accreditation with complete workflow traceability


FEATURES
The BD FACSDuet™ System delivers standardization, efficiency and flexibility

- Data reproducibility with standardization of processes and protocols
- Consistency in operations with an intuitive software interface minimizing training and variations due to different operators’ experience. System automation eliminates manual steps and the risk of generating errors and testing repetition.
- Efficiency and productivity with predictable sample throughput; the continuous specimen loading enables predictable volume processing with creation of lab test volume capacity.
- Traceability of specimen, sample, reagents and worklists eliminates manual data transcription and risk for error, helping conform to ISO 15189 accreditation
- Modularity and flexibility to accommodate a wide range of lab needs
Find out more from the BD FACSDuet™ System brochure
The BD FACSDuet™ System enables flexibility in specimen and tube loading

The BD FACSDuet™ Sample Preparation System answers the growing needs of laboratories to handle different types and sizes of specimen tubes and supports hospitals’ needs for consolidation and testing centralization.
The BD FACSDuet™ Sample Preparation System delivers the flexibility that is required by supporting nineteen (19) different types of blood collection tubes from different manufacturers, including BD Vacutainer®, Sarstedt S-Monovette™, Greiner and Streck Tubes.
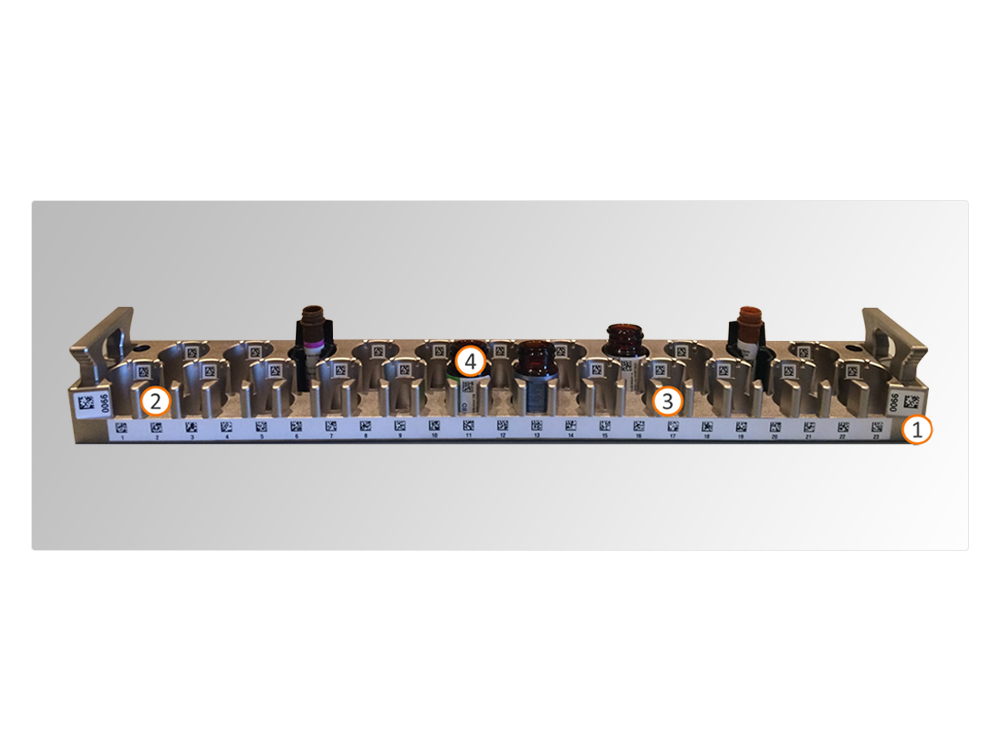
Specimen management is driven by tube adaptors. Seven (7) tube adaptors are available; they are barcoded and color coded for easy identification; they guarantee accurate tube piercing and sample volume dispensing.
Up to 40 specimen tubes can be accommodated at any given time in four racks holding 10 specimens each, driving workflow efficiency and throughput.
Two types of specimen racks are available, one of which is specifically designed for pediatric tubes.
Specimen traceability and automated worklist creation are guaranteed by five different barcodes on the specimen rack, identifying: (1) rack, (2) tube position, (3) empty position, (4) adaptor, (5) specimen.
Automated barcode scanning during rack insertion enables
- Patient specimen traceability
- Pipetting management
- Retrieving patient and assay information from the laboratory information system (LIS) for automated worklist creation
The BD FACSDuet™ System allows for sample throughout and traceability of samples and worklist through carrier barcode

Worklist traceability is enabled by scanning the carrier barcode, which is associated to a specific worklist and defined specimens.
Secondary tubes are loaded in the BD FACSDuet™ Sample Preparation System onto 30 or 40 tube sample carriers, compatible with the BD FACSLyric™ Flow Cytometer.
Barcoded secondary tubes are scanned to ensure sample traceability and association to the specimen and the worklist.
Up to three carriers can be loaded at the same time onboard the BD FACSDuet™ Sample Preparation System, allowing for increased sample throughput.
Refer to the technical specifications for more information.
The BD FACSDuet™ Sample Preparation System delivers flexibility in antibody panel design and walkaway capabilities by accommodating up to 46 reagent vials in two separate racks

The system enables a wide variety of reagent vials with the use of vial adaptors.
In addition to the barcode on the antibody vial (4), the reagent rack has 3 barcodes: identifying the rack (1), identifying the reagent position (2), and identifying the empty position (3). Barcodes on reagent racks and antibody vials drive reagent traceability, efficient antibody management and reduction of reagent waste.
Reagent cocktailing functionality allows on-board automated antibody cocktail preparation, eliminating the risk for errors in manual pipetting and driving automated recording of all used antibodies for easier tracking and auditing.
Cap racks are available to avoid misplacing caps and antibody cross-contamination.
Testimonials
Listen to the testimonial from Dr. Timothy Farren, Head of Immunophenotyping, Barts Heath NHS Trust, talking about how the BD FACSDuet™ System will help with ISO 15189 compliance.

“The automation allows faster process, faster throughput, increased capacity”,
adds Nicole Gresham,
Associate Director of Pathology, Barts Health NHS Trust, UK.

“BD FACSDuet™ Sample Preparation System—a real game changer in flow cytometry,”
says Mr. Chris Scott,
Lead Biomedical Scientist, Immunology Department, Barts Health NHS Trust, UK

“The integrated BD FACSDuet™-BD FACSLyric™ System satisfies our requirements of increasing efficiency, standardizing processes and improving quality.”
—Mrs. Debbie Bruno,
CD4 Laboratory Manager, Ampath Laboratories, South Africa

“I require all the monoclonal antibodies used, the ancillary reagents, tubes, the QC materials to be linked to an exact patient…this is an essential laboratory process and it is also mandatory in order to maintain ISO 15189 accreditation."

“I require all the monoclonal antibodies used, the ancillary reagents, tubes, the QC materials to be linked to an exact patient…this is an essential laboratory process and it is also mandatory in order to maintain ISO 15189 accreditation."
“The BD FACSDuet™ Sample Preparation System enables us to increase our laboratory’s efficiency and throughput, replacing manual processes with automated ones that are traceable and that can be automatically transferred and recorded in our laboratory information system providing a complete audit trail." “What is the potential impact of the BD FACSDuet™ Sample Preparation System? It removes our manual processes, increases our efficiency and throughput, and gives us control in standardization of processes. It’s a controlled environment.”
“The BD FACSDuet™ Sample Preparation System enables us to increase our laboratory’s efficiency and throughput, replacing manual processes with automated ones that are traceable and that can be automatically transferred and recorded in our laboratory information system providing a complete audit trail." “What is the potential impact of the BD FACSDuet™ Sample Preparation System? It removes our manual processes, increases our efficiency and throughput, and gives us control in standardization of processes. It’s a controlled environment.”
“At last, a fully integrated end-to-end walkaway solution, which is going to make our lives significantly easier. It reduces errors in both the pre-analytical and analytical phases, leading to increased productivity and efficiency, but most importantly provides a comprehensive fully auditable account of the whole process. Finally, a solution that creates capacity to allow us to meet our key performance indicators.”

“At last, a fully integrated end-to-end walkaway solution, which is going to make our lives significantly easier. It reduces errors in both the pre-analytical and analytical phases, leading to increased productivity and efficiency, but most importantly provides a comprehensive fully auditable account of the whole process. Finally, a solution that creates capacity to allow us to meet our key performance indicators.”
“I require all the monoclonal antibodies used, the ancillary reagents, tubes, the QC materials to be linked to an exact patient…this is an essential laboratory process and it is also mandatory in order to maintain ISO 15189 accreditation."

“I require all the monoclonal antibodies used, the ancillary reagents, tubes, the QC materials to be linked to an exact patient…this is an essential laboratory process and it is also mandatory in order to maintain ISO 15189 accreditation."
“The BD FACSDuet™ Sample Preparation System enables us to increase our laboratory’s efficiency and throughput, replacing manual processes with automated ones that are traceable and that can be automatically transferred and recorded in our laboratory information system providing a complete audit trail." “What is the potential impact of the BD FACSDuet™ Sample Preparation System? It removes our manual processes, increases our efficiency and throughput, and gives us control in standardization of processes. It’s a controlled environment.”

“The BD FACSDuet™ Sample Preparation System enables us to increase our laboratory’s efficiency and throughput, replacing manual processes with automated ones that are traceable and that can be automatically transferred and recorded in our laboratory information system providing a complete audit trail." “What is the potential impact of the BD FACSDuet™ Sample Preparation System? It removes our manual processes, increases our efficiency and throughput, and gives us control in standardization of processes. It’s a controlled environment.”
“At last, a fully integrated end-to-end walkaway solution, which is going to make our lives significantly easier. It reduces errors in both the pre-analytical and analytical phases, leading to increased productivity and efficiency, but most importantly provides a comprehensive fully auditable account of the whole process. Finally, a solution that creates capacity to allow us to meet our key performance indicators.”

“At last, a fully integrated end-to-end walkaway solution, which is going to make our lives significantly easier. It reduces errors in both the pre-analytical and analytical phases, leading to increased productivity and efficiency, but most importantly provides a comprehensive fully auditable account of the whole process. Finally, a solution that creates capacity to allow us to meet our key performance indicators.”
“I require all the monoclonal antibodies used, the ancillary reagents, tubes, the QC materials to be linked to an exact patient…this is an essential laboratory process and it is also mandatory in order to maintain ISO 15189 accreditation."

“I require all the monoclonal antibodies used, the ancillary reagents, tubes, the QC materials to be linked to an exact patient…this is an essential laboratory process and it is also mandatory in order to maintain ISO 15189 accreditation."


-
Brochure
-
Technical Specifications
-
Product List
-
Data

Request a Quote
Please fill in the following information and we will get in touch with you regarding your query.
The BD FACSDuet™ Sample Preparation System is a Class 1 Laser Product.
The BD FACSDuet™ Sample Preparation System is CE marked in compliance with the European In Vitro Diagnostic Medical Device Directive 98/79/EC. Sample preparation for user-defined protocols and cocktailing functions have not been validated for IVD use and require validation by the user.
Unless otherwise noted the products are for in Vitro Diagnostic Use. CE marked in compliance with the In Vitro Diagnostic Medical Device Directive 97/79/EC
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.