Old Browser
For Professionals in Clinical Diagnostics
Discover the BD FACSLyric™ Flow Cytometry System difference.
- Witness clinical performance results you have never seen before through high sensitivity and improved resolution
- Streamline your lab workflow through flexibility and automation, enabling efficiency and productivity
- Achieve automated standardization through highly reproducible results and enable collaboration through assay portability
- See how the BD FACSLyric™ System can transform your lab.
Get more information from the BD FACSLyric™ Flow Cytometry System Brochure.


- Chapters
- descriptions off, selected
- subtitles settings, opens subtitles settings dialog
- subtitles off, selected
This is a modal window.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
Features
Discover the BD FACSLyric™ Flow Cytometry System—A Next-Generation Flow Cytometer
Built on a foundation of excellence, experience and expertise, the BD FACSLyric™ Flow Cytometry System is our next generation diagnostic standard for clinical cell analysis, transforming the way your lab does flow cytometry. As with all BD instruments, the BD FACSLyric™ Flow Cytometry System is backed by 40 years of BD expert training, service and support—so there’s no limit to your potential.
The Power of the BD FACSLyric™ Flow Cytometer
- 4-, 6-, 8-, 10- and 12-color configurations. Onsite upgradeable to adapt to your lab's changing needs
- Up to 3 lasers—blue, red and violet—12 fluorescence channels and 14 parameters
- 35,000 events per second maximum acquisition rate; no limit on number of events acquired
- Automated single-tube QC with BD® CS&T Beads
- Fluorescence compensation required only every 60 days, improving efficiency and productivity
- 21 different loading options: plates or tubes; built-in flexibility with BD FACS™ Universal Loader
- Powered by BD FACSuite™ Clinical Software for Acquisition and Analysis, supports U.S, FDA 21 CFR Part 11 compliance offering password protection, audit trail, electronic signatures and IQ/OQ procedures
- BD FACSLink™ Laboratory Information System (LIS) Interface and BD Assurity Linc™ Remote Systems Management Software streamline your workflow and help improve productivity through seamless integration.
See how you can simplify your workflow using the automated instrument setup and compensation on the BD FACSLyric™ System.


Performance
A new diagnostic standard delivering outstanding performance and standardization within and between instruments
The BD FACSLyric™ Flow Cytometry System is a high-performance, highly sensitive flow cytometer that demonstrates exceptional resolution and improved separation to make dim and rare cell populations easier to resolve.
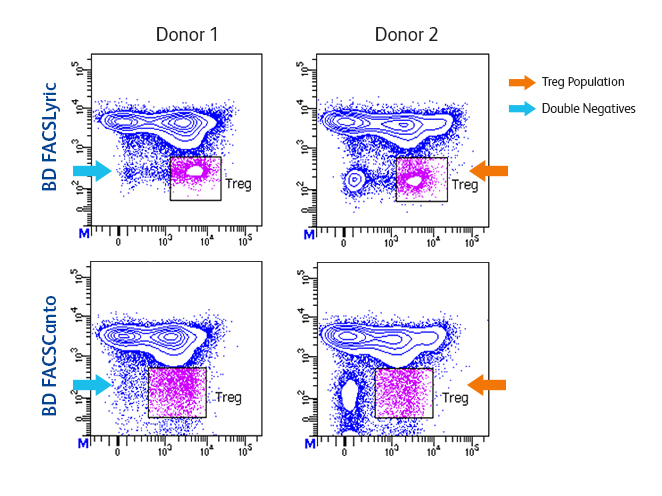
Higher sensitivity makes dim and rare populations easier to resolve.
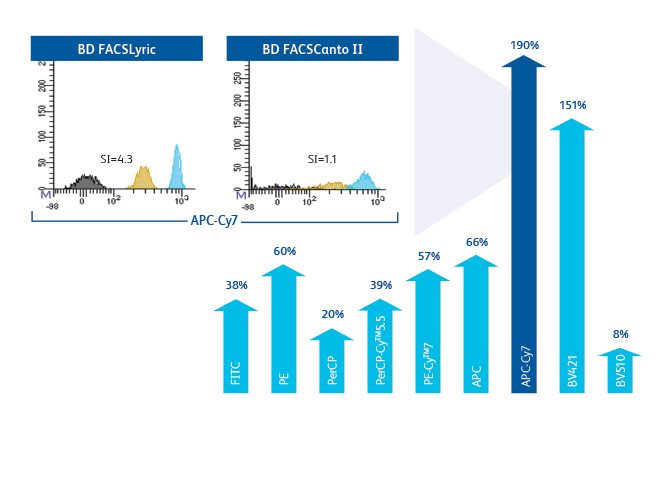
Improvement in stain index of 8–190% across all parameters ensures better separation and enables faster analysis and easier gating.
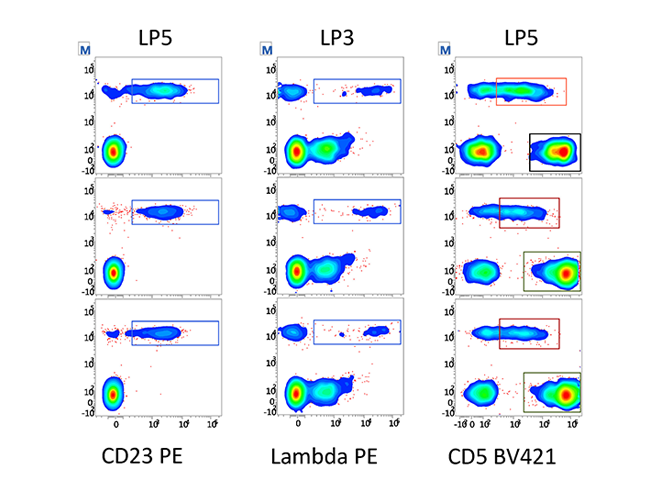
Assay portability simplifies and standardizes instrument setup within your lab and between labs. It also enables the sharing and exchange of data, ideas, and IVD and user-defined protocols within and between institutions.
Highly reproducible results between instruments drive standardization.
| Blue Laser | %CV |
| FITC | 4.2 |
| PE | 4.4 |
| PE-Cy7 | 4.8 |
| PerCP | 10.2 |
| PerCP-Cy5.5 | 7.9 |
| Red Laser | %CV |
| APC | 5.3 |
| APC-Cy7 | 4.1 |
| APC-H7 | 3.9 |
| APC-R700 | 4.2 |
| Violet Laser | %CV |
| BD Horizon™ V450 | 11.1 |
| BD Horizon™ V500 | 11 |
| BD Horizon™ BV605 | 13.6 |
| BD Horizon™ BV711 | 7.5 |
| BD Horizon™ BV786 | 15.3 |
Between-instrument reproducibility of target MFI values on the BD FACSLyric™ System
Lyse/wash assay settings were imported across 15 instruments to show effects of standardization on beads. The CVs of the fluorescence intensity across all channels varies by less than 15.3%. Daily QC with one lot of BD® Cytometer Setup & Tracking Beads was run on fifteen BD FACSLyric™ Flow Cytometers. For each instrument, the PMTV gains were automatically adjusted to meet the target values. BD® FC Beads acquired on each BD FACSLyric™ Instrument. The MFI of positive populations was measured for all parameters across all instruments. The %CV is shown. The data for this internal study were acquired using BD® FC Beads across 15 BD FACSLyric™ Instruments. Greater between-instrument variability could be observed when running biological samples, when using non BD reagents or when comparing fewer instruments.
Applications
A Powerful Solution for CD4 Counts and Other Immunological Assessment
With the BD FACSLyric™ Flow Cytometer, clinical laboratories can obtain repeatable and reliable results for immunological assessment of patients having or suspected of having an immune deficiency such as HIV.
BD Multitest™ Reagents, formulated to be used with BD Trucount™ Tubes, provide absolute counting capability.
The BD FACSuite™ Clinical Application, with predefined templates for 4- and 6-color T, B and/or NK cell analysis, provides reproducible and consistent results.
Each reagent includes a cocktail of multiple fluorescently labeled monoclonal antibodies, premixed at the appropriate titer to ensure quality staining.

A streamlined methodology for immunological assessment of patients having or suspected of having an immune deficiency such as HIV using the BD FACSLyric™ Flow Cytometer.
-
Brochure

Request a Quote
Please fill in the following information and we will get in touch with you regarding your query.
Omana-Zapata I, Mutschmann C, Schmitz J, et al. Accurate and reproducible enumeration of T-, B- and NK lymphocytes using the BD FACSLyric 10-Color System: a multisite clinical evaluation. PLoS One. 2019;14(1):e0211207. doi: 10.1371/journal.pone.0211207.

Class 1 Laser Product Cy™ is a trademark of GE Healthcare.
Cy™ dyes are subject to proprietary rights of GE Healthcare and Carnegie Mellon University, and are made and sold under license from GE Healthcare only for research and in vitro diagnostic use.
Any other use requires a commercial sublicense from GE Healthcare, 800 Centennial Avenue, Piscataway, NJ 08855-1327, USA. The BD FACSLyric™ Flow Cytometer is for In Vitro Diagnostic Use with BD FACSuite™ Clinical Application for up to 6 colors. 7 to 12 colors are for Research Use Only.
BD FACSuite™ Clinical Application is for In Vitro Diagnostic Use. BD FACSuite™ Application is for some IVD, ASR and RUO products only.
Refer to manufacturer's instructions for use and related User Manuals and Technical Data Sheets before using this product as described.
Comparisons, where applicable, are made against older BD technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.
