Overview
The fusion of safety, performance and sorting
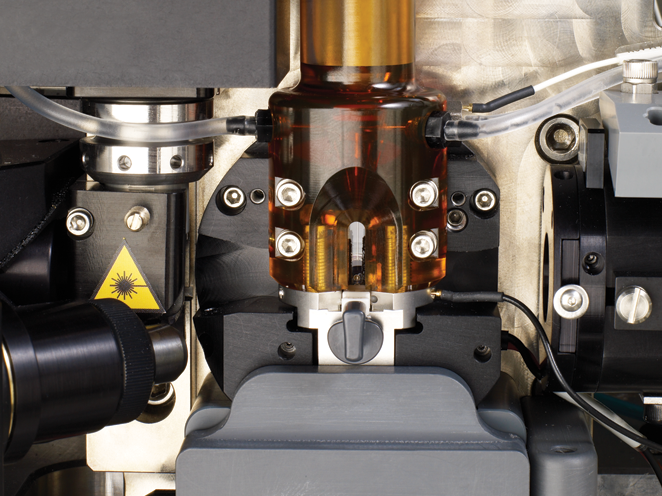
The BD FACSAria™ Fusion Flow Cytometer combines sorting capabilities with best-in-class biosafety expertise for a comprehensive advanced cell sorter and biosafety solution.
The fully integrated biosafety cabinet meets emerging operator and sample protection requirements as well as many country standards for bioprotection. The instrument uses BD FACSDiva™ Software to efficiently control the setup, acquisition and analysis of flow cytometry data from the operator workstation. The software is common across many BD cell analyzers and cell sorters, making the BD FACSAria™ Fusion Flow Cytometer compatible with them.
Get more information from the BD FACSAria™ Fusion Flow Cytometer brochure.
FEATURES
PERFORMANCE
The BD FACSAria™ Fusion Flow Cytometer provides sensitivity for multicolor and sorting applications
Sensitivity to resolve dim staining populations
The lasers are specifically selected for optimal signal-to-noise separation and improve the resolution of dim populations, thereby enabling more effective gating for sorting. Set at peak performance, the lasers enable dim staining populations to fluoresce more brightly, facilitating population resolution and enabling subsequent gating for sorting.
APPLICATIONS
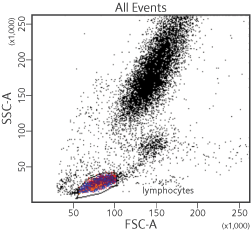
The BD FACSAria™ Fusion System has been used in resolving various types of cell populations

-
Brochures
-
Filter Guides
-
Frequently Asked Questions
-
Quick Reference Guides
-
Job Aids
-
Technical Specifications
-
Test Reports
-
Webinars
-
Cross-Instrument and Cross-Site Standardization Using BD FACSDiva Software's Custom Application Settings: Part II of II
-
Instrument Characterization and Performance Tracking for Digital Flow Cytometers
-
Using the BD® Cytometer Setup and Tracking (CS&T) System for Instrument Characterization and Performance Tracking
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Class 1 Laser Product.
Cy™ is a trademark of GE Healthcare. Cy dyes are subject to proprietary rights of GE Healthcare and Carnegie Mellon University and are made and sold under license from GE Healthcare only for research and in vitro diagnostic use. Any other use requires a commercial sublicense from GE Healthcare, 800 Centennial Avenue, Piscataway, NJ 08855-1327, USA.
CF™ is a trademark of Biotium, Inc.
Alexa Fluor® and Pacific Blue™ are trademarks of Thermo Fisher Scientific.